Wednesday Feb 25, 2026
Wednesday Feb 25, 2026
Tuesday, 27 April 2021 01:01 - - {{hitsCtrl.values.hits}}
With the spike in COVID-19 cases signalling a probable third wave, private sector hospitals are renewing their appeal to the Government to let them import and administer vaccines as per the pre-set guidelines with due State supervision.
Since the availability from early January, the Government has been the sole importer of the COVID-19 vaccine.
At present, PCR tests can be done by accredited private hospitals which also run treatment centres.
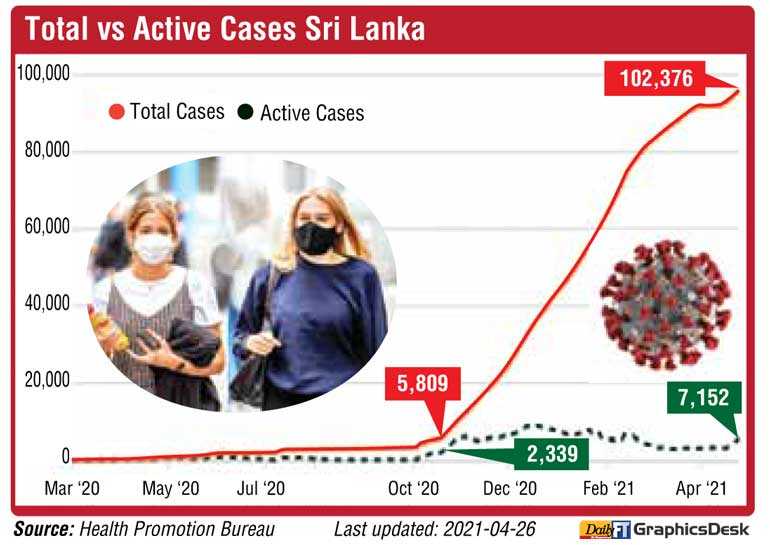
Yesterday 997 persons tested positive increasing the number of active cases to 7,152 and cumulative figure from recent clusters to 96,698 up by 3,546 from 93,152 as of end Thursday. The overall total of COVID-19 cases stood at 102,376 of which over 95,000 had recovered. The number of deaths was 647.
The private sector had suggested that of its imports, it could set aside a pre-specified amount for the Government’s free immunisation drive.
The fresh call to let the private sector participate in the vaccination program follows India, which yesterday in a big step to accelerate the pace of vaccination permitted anyone above 18 to receive anti-COVID shots from 1 May, opening options for hospital chains as well as corporate houses to procure and vaccinate all eligible recipients.
The decision was taken at a meeting chaired by Prime Minister Narendra Modi, which noted that satisfactory coverage of vulnerable groups currently eligible for vaccination was expected by 30 April, setting the stage for a more liberalised approach to inoculation against COVID-19 in terms of pricing as well as age-determined eligibility.
India allowed pharmaceutical manufacturing companies to sell 50% of their production to states and hospital chains at a pre-fixed price for vaccination of people above 18 outside the central program.
States will be free to decide the age bar for any category of people above 18 (for example a particular state may adopt the 18-plus norm or set an age bar of its choosing).
The Times of India reported that Indian States and hospital chains will be allowed to procure vaccines directly from manufacturers and even contract them from abroad from candidates that are approved for use by the Centre under its recently-announced policy where shots approved by well-known regulators like FDA and MHRA or those listed with the WHO can be given emergency approval in India.
The effort, keeping in mind a plateauing and a dip in daily vaccination, is to boost the pace of inoculation by liberalising procurement and widening eligibility, it added.
Since its roll out on 29 January, Sri Lanka’s nation-wide immunisation program has covered 925,242 people. On Friday, the Government claimed that it was on track to immunise 40% of the population by September.
SPC Chairman Dr. Prasanna Gunasena said agreements between vaccine manufacturers and the State Pharmaceuticals Corporation (SPC) would guarantee the country 2.6 million doses of Sputnik V vaccines by July and 4.94 million doses of Pfizer vaccines by December.
He said that agreements had been signed with manufacturers of both vaccines to receive a set number of doses within a specified timeline.
Accordingly, 35,000 doses of Pfizer vaccines are expected between April and June; 105,000 doses are expected between July and September; and 4.8 million doses are expected between October and December.
The timeline for Sputnik V as detailed in the agreement guarantees 200,000 doses in April, 400,000 doses in May, 800,000 doses in June and 1.2 million doses in July.
Funding for the vaccines has been secured, with the Pfizer vaccines funded by the World Bank and the Asia Development Bank (ADB) and the Government covering the cost of other vaccines.