Sunday Feb 22, 2026
Sunday Feb 22, 2026
Saturday, 6 February 2021 00:00 - - {{hitsCtrl.values.hits}}
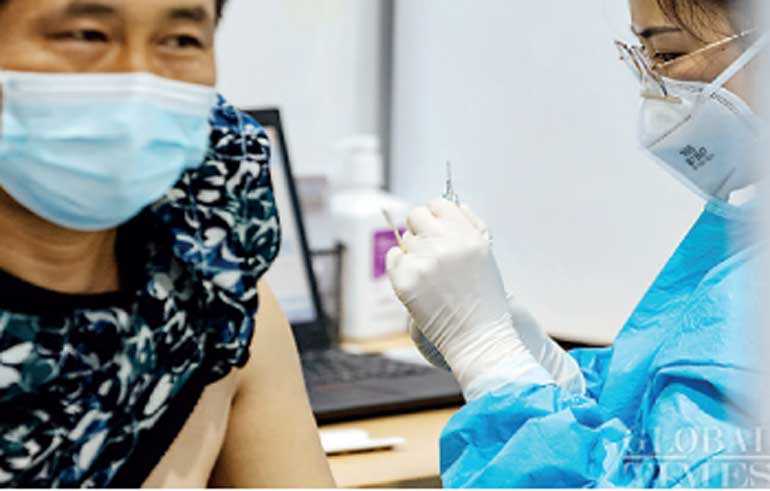
China has administered over 31 million doses of COVID-19 vaccines for key groups as of Wednesday, Mi Feng, spokesman for the National Health Commission, said at a media briefing on Thursday.
China granted conditional market approval for its first COVID-19 vaccine on 30 December 2020, while vaccines in the last stage of clinical trials have been available for emergency use in the country since last June. By the end of 14 December, COVID-19 vaccines developed by China were under clinical trials, including five in phase 3, the final trial before a vaccine is approved for market.
The inactivated COVID-19 vaccine, developed by State-owned Sinopharm, which won conditional market approval, is safe and has an efficacy of 79%, the company said.