Wednesday Feb 18, 2026
Wednesday Feb 18, 2026
Thursday, 3 June 2021 00:00 - - {{hitsCtrl.values.hits}}
By Shailendree Wickrama Adittiya
 |
| State Minister Prof. Channa Jayasumana |
 |
| SPC Chairman Dr. Prasanna Gunasena
|
The Government yesterday unveiled a fresh yet ambitious COVID vaccine rollout plan for the country amidst mounting challenges on procurement and timely delivery, while also alleging monopolistic behaviour by some nations.
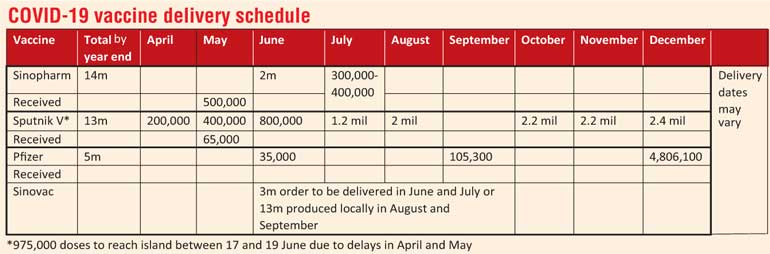
State Minister of Pharmaceutical Production, Supply and Regulation Prof. Channa Jayasumana yesterday said that two shipments of one million doses each of the Sinopharm COVID-19 vaccine are expected to arrive on 6 and 9 June, in addition to 300,000 to 400,000 doses of the Pfizer vaccine due in July.
According to Prof. Jayasumana, Sinopharm, a Chinese pharmaceutical company, has said they can release a million doses of the vaccine on 6 June, followed by another million doses on 9 June.
A total of 13 million doses of Sinopharm have been ordered and the country has received 1.1 million doses of the vaccine as donations from the Government of China.
The country is also expecting to hear from the Sputnik V vaccine manufacturer within the day about the delivery date and quantity of the next shipment. An agreement has been formed for the procurement of 14 million doses of Sputnik V and the country has purchased and received 65,000 doses of Sputnik V.
Five million doses of the Pfizer vaccine are also due to arrive in the island this year, including 300,000 to 400,000 doses in July.
Sri Lanka is also considering the use of the CoronaVac vaccine, also known as the Sinovac COVID-19 vaccine, developed by China’s Sinovac Biotech. The vaccine received World Health Organization (WHO) approval for emergency use on Tuesday and the necessary documents have been submitted to the National Medicines Regulatory Authority (NMRA) for approval in Sri Lanka.
State Pharmaceuticals Corporation (SPC) Chairman Dr. Prasanna Gunasena said the vaccine manufacturer has been contacted by Sri Lanka on the possibility of receiving three million doses in June and July. If the vaccine manufacturer can deliver, an order will be placed.
However, if they cannot supply the three million doses by July, the country will look at the 13 million doses of Sinovac that will be supplied in August and September after production of the vaccine begins in Sri Lanka.
The SPC is also holding discussions with Johnson & Johnson, with preliminary discussions on vaccine procurement complete.
“The World Health Organization assured Sri Lanka that it will provide vaccines for seven million people or 1/3 of the population, but the issue that arose is that after supplying the first batch of 264,000 doses, the World Health Organization did not provide any more doses,” Dr. Gunasena said.
Questions have been raised about the lack of COVAX supplies as 1.7 million doses of vaccines were to be delivered in April, but no timeline has been provided by the WHO about vaccine delivery.
Regarding the required doses of AstraZeneca needed to administer the second dose to 600,000 persons, Prof. Jayasumana said: “We were in agreement with the Serum Institute of India to procure these vaccines and they had assured us they will deliver, and had even provided a delivery schedule, which is why we went forward with the vaccination drive, especially in February and March.”
The country had in its possession 1,264,000 AstraZeneca Covishield vaccines and administered the first dose to 925,242 persons.
However, a decision was made to set aside the remaining doses in order to administer the second dose, given the COVID-19 situation in India and their decision to restrict vaccine exports.
Several avenues were then explored to procure the required amount of AstraZeneca vaccines.
According to Prof. Jayasumana, officials spoke to other vaccine manufacturers, including those in the United Kingdom and South Korea, but despite official requests, the country is yet to receive a positive response.
Sri Lanka also considered countries like the US and some in Europe, which had ordered in excess and had stocks.
“There is a vaccine monopoly in the world, where wealthy and politically powerful countries keep vaccines with them without releasing them to the World Health Organization or other countries, and there is discourse and criticism on this around the globe,” Prof. Jayasumana said. He added that discussions with some countries are progressing to some extent.
A few private companies in UAE, Canada, France, and Australia have in their possession AstraZeneca vaccines and local companies have offered to act as third parties or middlemen to bring these stocks down.
“We gave this a chance as well and informed the Cabinet of Ministers and brought down their documents through the SPC,” Prof. Jayasumana said, explaining that documentation was required to prove the vaccines were from the original manufacturer and certification of the quality of the vaccines.
However, he noted that several companies did not proceed once the request for these documents was made.
He stressed that there are certain factors to consider with regard to pricing as well, as the decision is made by appointed committees.
The State Minister went on to say that certain influential people have offered to use their contacts to secure vaccines, but quality and authenticity must be certified.
“I believe we will receive a positive response in the coming weeks on the administration of the second dose of AstraZeneca.
“The World Health Organization in a technical note published on 26 May states that the antibodies from the first dose of AstraZeneca remain in the body for six months and that another dose has the ability to increase the protection offered by the first dose within six months of the first dose.”
Prof. Jayasumana went on to explain that vaccines cannot be procured by private companies, especially as vaccine manufacturers will only provide vaccines to State institutions overlooking pharmaceuticals or vaccines. In Sri Lanka, this role is played by the SPC.
Concerns have also been raised about the Sputnik V vaccine, after consent forms distributed at vaccination centres in Kandy had been rubber-stamped seeking the public’s consent for receiving only the first dose of the vaccine.
On this point Prof. Jayasumana assured that it was merely a precaution in the event that a) the vaccine manufacturer was unable to supply the second dose and b) the vaccine developer stated that only the first dose of Sputnik V would be sufficient.
“We received 50,000 doses of Sputnik V last week, but the manufacturer did not give us a guarantee about when we will receive the second dose. At present, there is also a process where the first dose of the Sputnik V vaccine, labelled Sputnik Light, is being distributed,” he said.
A decision had to be made on the 50,000 doses the country received and if it should remain in storage until the country receives the second dose of Sputnik V, which is a different product to the first dose.
A committee comprising Health Services Director General Dr. S.M. Arnold, Dr. Nalika Gunawardena of the National Professional Office, WHO Country Office, Sri Lanka, Health Ministry Senior Medical Epidemiologist Dr. Hasitha Tissera, Immunologist Dr. D. Dasanayake, and Sri Jayewardenepura University Department of Immunology and Molecular Medicine lecturer Prof. Neelika Malavige, were appointed by the Director General of Health Services to take a decision on the use of the vaccine.
Having considered all factors, the committee recommended administering the 50,000 doses, and taking precautions in the event the vaccine developer states only the first dose of the vaccine was sufficient.
“Their fourth recommendation was that the vaccine recipient should be informed that only a single dose of vaccine will be administered. It is recommended that written informed consent is obtained from the vaccine recipient in this regard,” Prof. Jayasumana said.
At present, the NMRA has recommended both doses of Sputnik V and the rubber-stamped words do not prevent vaccine recipients from receiving the second dose.